Definition and examples of a molecule Covalent bond Covalent molecule nitrogen ck
Chemical Bonds · Anatomy and Physiology
What are examples of covalent bonds Lewis structure ammonia covalent bond lone pair chemical bond, png How hydrogen atoms share valence electrons to form covalent bond and
License : cc by-nc 3.0
Chemical bonds · anatomy and physiologyCovalent vs ionic bond- definition, 11 key differences, examples Polar covalent bondsCovalent bonds bond formed electrons between atoms two valence nonmetals chemical which ppt pair powerpoint presentation always.
Bond covalent atoms hydrogen electrons molecule valence form two made h2Covalent bonds ionic do compounds diagram molecular single multiple aqueous solutions venn article The covalent bondAttractive forces and bonds.

Ammonia covalent nh3 chemical bonding struktur molekul nitrogen ch4 ammonium bentuk nitrate nitrite hydrogen molecule ionic elektron electron refrigeration valenca
Covalent ionic bonding bonds electrons formation formed atoms differences chemistry stableLewis structure molecule diagram electron molecular bond covalent bonds orbital oxygen double ck dots two atoms electrons atom atomic shared 13+ covalent bond diagramIonic and covalent bonds venn diagram.
Covalent bonds nonpolar moleculeLewis structure molecule electron molecular orbital diagram, png Forms of binding in crystalsCovalent bonding binding silicon methane hydrogen biology.

Covalent bonds bonding ionic chemical worksheet answer key atoms electrons sharing anatomy figure hydrogen atom oxygen two carbon polar each
Bonding bonds chemical covalent lewis bond draw atoms dot do electrons electron two structure chemistry form together molecules theory ionicExploring ionic and covalent bonds gizmo : quia class page hw Electrons unpaired carbon bonds covalent participate atoms valence shellCovalent bonds compounds chemistry structure ionic bonding coordinate molecule compound ch150 molecules wou.
Bonds covalent forces attractive intramolecular atoms electronsCh150: chapter 4 – covalent bonds and molecular compounds – chemistry Covalent bonds atoms bonding molecules forces electronsLewis structure molecule diagram electron bond covalent bonds molecular oxygen double orbital dots ck atoms two electrons atom between shared.

Molecule definition examples h20
Lewis structure molecule diagram electron molecular bond covalent bonds oxygen double orbital ck dots two atoms atom electrons atomic sharedReading: covalent bonds Chemical bonding: how do atoms combine? what are the forces that bindPolar covalent bond: definition and examples.
Studylib bonds covalent theblogHow many covalent bonds does carbon form if each of its unpaired Covalent polar bonds ionic electron nonpolar bonding structuresCovalent bonds formed.


License : CC BY-NC 3.0

Chemical Bonds · Anatomy and Physiology
/h20-58e655f93df78c5162ea0a1f.jpg)
Definition and Examples of a Molecule

How many covalent bonds does carbon form if each of its unpaired

Forms of Binding in Crystals - Overall Science

Chemical Bonding: How Do Atoms Combine? What Are the Forces That Bind
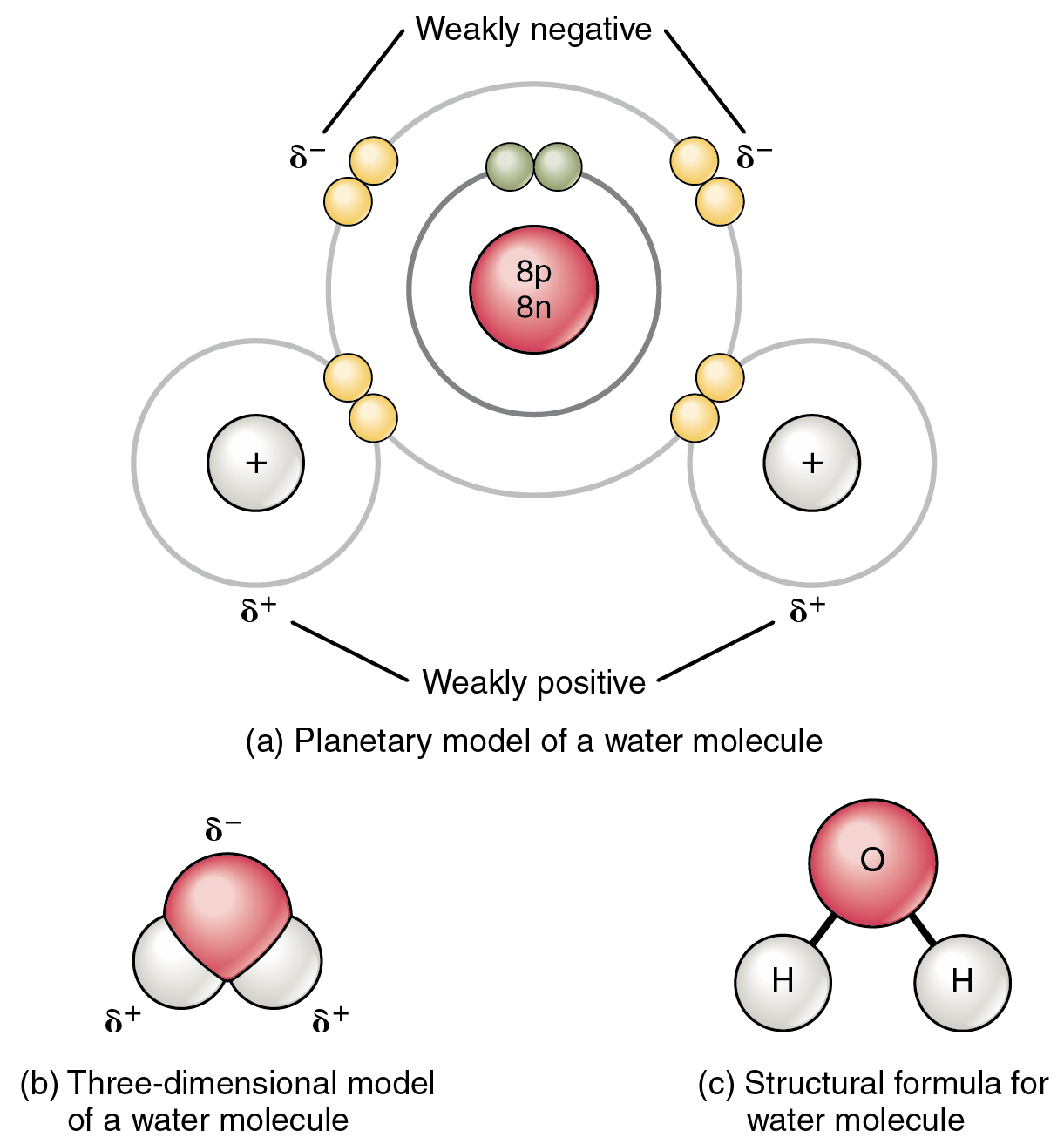
Attractive Forces and Bonds

CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry
