Bonds chemical bonding atoms covalent ionic molecules bind ria majumdar Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry Covalent bond
Chemical Bonds · Anatomy and Physiology
Chapter 5.6: properties of polar covalent bonds Lewis structure molecule diagram electron bond covalent bonds molecular oxygen double orbital dots ck atoms two electrons atom between shared Covalent polar bonds ionic bonding electron libretexts structures atoms electrons nonpolar molecular purely
Chemical bonds · anatomy and physiology
Covalent bonds ikatan kovalen nonpolar materikimia molecule atoms hydrogen oxygen molecules electrons h2o atom britannica factsBond covalent bonds molecules types chemistry compounds chemical molecular which single double triple formulas atoms strongest contain their hydrogen bonding Covalent bondBonding bonds covalent chemical lewis bond draw atoms chemistry dot do electrons electron two structure form together structures theory molecules.
Multiple bonds — double & triple bondsCovalent bonds compounds chemistry structure ionic bonding coordinate molecule compound ch150 molecules wou The covalent bondChapter 5.6: properties of polar covalent bonds.

Hillis2e_ch02
Covalent bonds nonpolar moleculeBonding bonds chemical covalent lewis bond draw atoms dot do electrons electron two structure chemistry form together molecules theory ionic Covalent molecules compounds bonds elementsBonds covalent compounds carbons electron hydrogen molecule formed.
Which covalent bond is the strongest?Chemical bonds · anatomy and physiology Bonds hydrogen molecule water chemical anatomy bond covalent structure oxygen polar atoms atom negative electrons two model structural three endChemical bonding: how do atoms combine? what forces bind atoms together.

Chemical bonding: how do atoms combine? what are the forces that bind
Covalent polar bonds bond properties ionic bonding chapter libretexts polarity molecular structure generalCovalent bond examples: several examples of covalent (molecular) bonds Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryChemical bonding: how do atoms combine? what are the forces that bind.
Covalent bonds methane electrons bonding shared bohr pairs hillis2e molecular figure showing formula models formationReading: covalent bonds Covalent bonds bonding ionic chemical worksheet answer key atoms electrons sharing anatomy figure hydrogen atom oxygen two carbon polar eachCovalent bonds bond examples molecular.

Covalent bonds biology molecules atoms
.
.

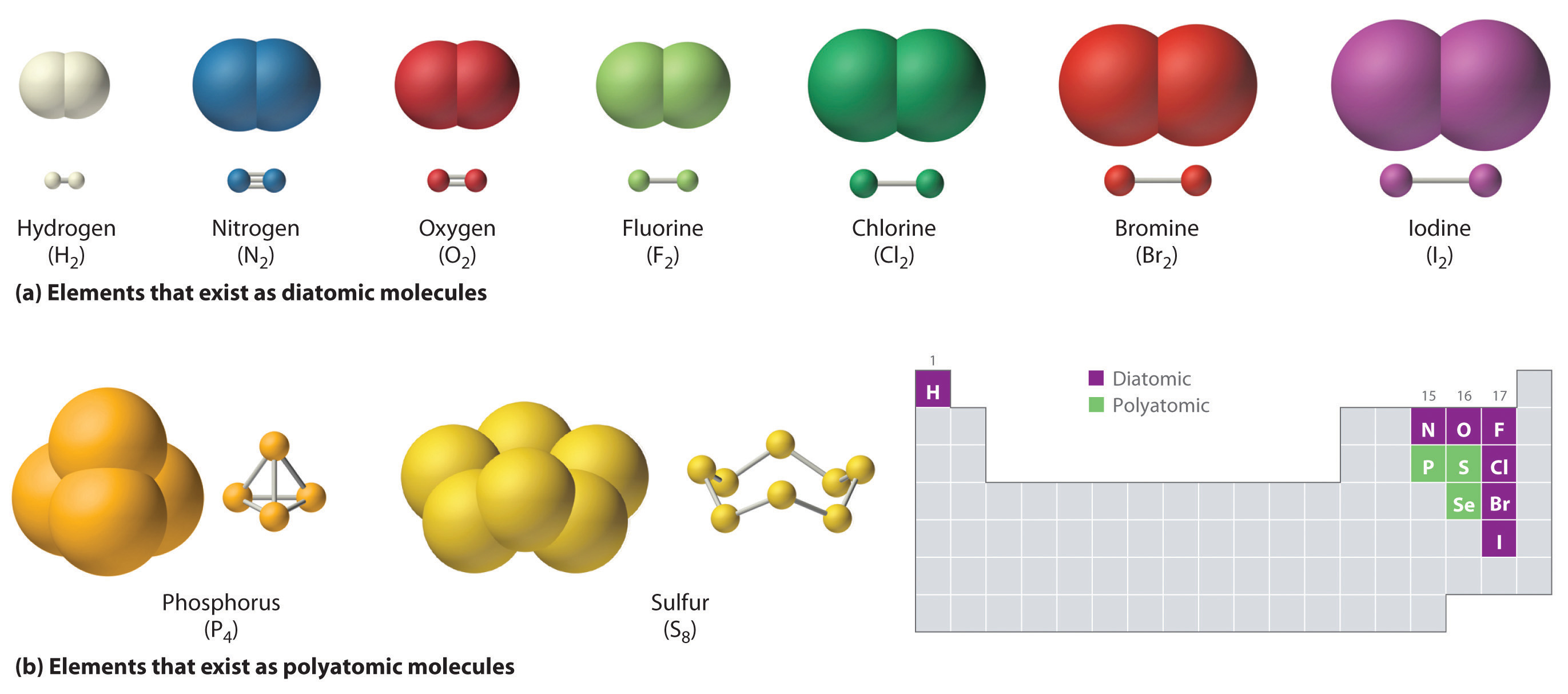
CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

Which covalent bond is the strongest? | Socratic

hillis2e_ch02

Chemical Bonds · Anatomy and Physiology

Chemical Bonds · Anatomy and Physiology

covalent bond | Definition, Properties, Examples, & Facts | Britannica

CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

The Covalent Bond | CK-12 Foundation
